-
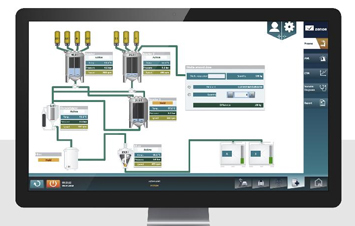
- Zenon for Pharmaceutical(Supervisor>Development, Runtime)
-
Limited 13만 태그, Unlimited 100만 태그 이상 적용사례 보유.
국제 규정 준수(International Regulation Compliance)
제약 제조업체가 구축하는 디지털화 시스템은 국가별 및 국제 관련 규정을 반드시 준수해야 합니다. FDA 21 CFR Part 11 및 EU GMP 가이드라인 Annex 11을 표준으로 충족하는 zenon으로 규정 준수에 필요한 노력을 최소화 할 수 있습니다. 두 규정 모두 전자 기록 및 컴퓨터 시스템의 적절한 사용을 규정하고 있습니다. zenon은 GAMP 5 Software Category 4 요건도 충족하는 구성 가능한 소프트웨어입니다.
GMP준수프로젝트구축의용이성
(GMP, Data Integrity Compliance, Data Integrity Regulation Compliance)
* 사용자관리(Authorization Management)
* 전자서명(eSignature)
* 감사추적(Audit Trail)
* 레시피관리(Recipe Management)
* 편차감지(Deviation detection)
* 히스토리안(Historian)
* 전자배치기록(Electronic Batch Report)
* 예외검토지원보고서(RBE)
* 데이터내보내기(Export Data)
* 보안조치 (Security measures)

Zenon for Pharmaceutical(Supervisor>Development, Runtime)


 home > 제품소개 >
home > 제품소개 > 
























